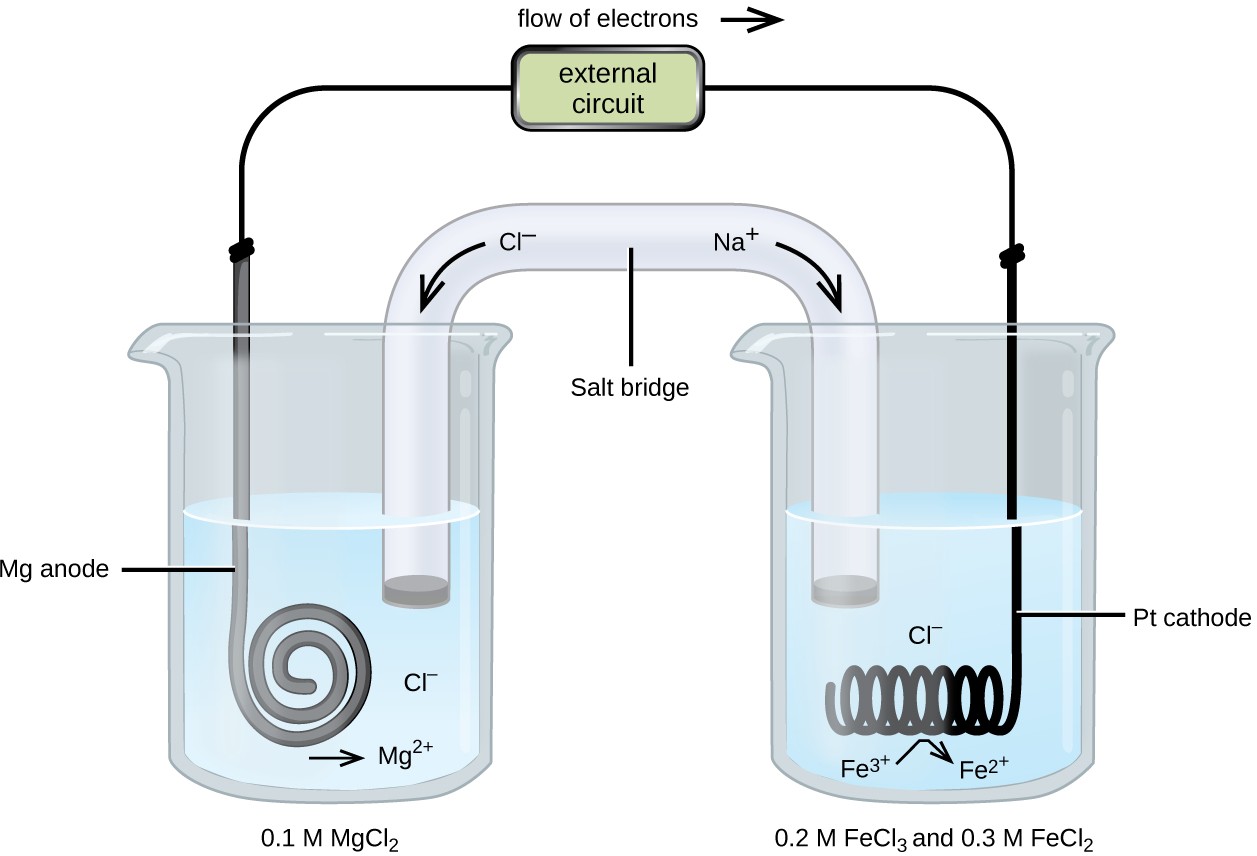
What Is The Function Of Zinc Electrode In A Galvanic Cell . Web a zinc sulfate solution is floated on top of the copper sulfate solution; Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Zinc ions in the solution are positive, and hence. Web electrons are flowing from the zinc solid metal strip to the copper strip. Then a zinc electrode is placed in the zinc sulfate solution. The salt bridge plays a very important role in a galvanic cell: Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the.
from psu.pb.unizin.org
Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Zinc ions in the solution are positive, and hence. Web electrons are flowing from the zinc solid metal strip to the copper strip. The salt bridge plays a very important role in a galvanic cell: Then a zinc electrode is placed in the zinc sulfate solution. Web a zinc sulfate solution is floated on top of the copper sulfate solution;
Galvanic Cells (17.2) Chemistry 110
What Is The Function Of Zinc Electrode In A Galvanic Cell The salt bridge plays a very important role in a galvanic cell: Web a zinc sulfate solution is floated on top of the copper sulfate solution; Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Zinc ions in the solution are positive, and hence. Then a zinc electrode is placed in the zinc sulfate solution. The salt bridge plays a very important role in a galvanic cell: Web electrons are flowing from the zinc solid metal strip to the copper strip. Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Zinc ions in the solution are positive, and hence. Then a zinc electrode is placed in the zinc sulfate solution. Web if we connect the zinc and copper by means of a. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working What Is The Function Of Zinc Electrode In A Galvanic Cell The salt bridge plays a very important role in a galvanic cell: Zinc ions in the solution are positive, and hence. Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web electrons are flowing from the zinc solid metal strip to. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Zinc ions in the solution are positive, and hence. The salt bridge plays a very important role in. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.pinterest.com
Difference Between Daniell Cell and Galvanic Cell Definition, How What Is The Function Of Zinc Electrode In A Galvanic Cell Web electrons are flowing from the zinc solid metal strip to the copper strip. Then a zinc electrode is placed in the zinc sulfate solution. Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web a zinc sulfate solution is floated. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper What Is The Function Of Zinc Electrode In A Galvanic Cell Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Web electrons are flowing from the zinc solid metal strip to the copper strip. Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free What Is The Function Of Zinc Electrode In A Galvanic Cell The salt bridge plays a very important role in a galvanic cell: Zinc ions in the solution are positive, and hence. Web electrons are flowing from the zinc solid metal strip to the copper strip. Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From chemistrypage.in
What is Electrochemistry Used for? Electrochemistry example What Is The Function Of Zinc Electrode In A Galvanic Cell The salt bridge plays a very important role in a galvanic cell: Web electrons are flowing from the zinc solid metal strip to the copper strip. Zinc ions in the solution are positive, and hence. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Web because the zinc electrode in this cell dissolves spontaneously to. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. Zinc ions in the solution are positive,. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in What Is The Function Of Zinc Electrode In A Galvanic Cell Zinc ions in the solution are positive, and hence. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From alevelchemistry.co.uk
Redox and Electrode Potential ALevel Chemistry Revision Notes What Is The Function Of Zinc Electrode In A Galvanic Cell Web a zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge plays a very important role in a galvanic cell: Zinc ions in the solution are positive, and hence. Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From nikhilnandepu97.blogspot.com
Nikhil Nandepu Galvanic Cell Construction What Is The Function Of Zinc Electrode In A Galvanic Cell Web electrons are flowing from the zinc solid metal strip to the copper strip. The salt bridge plays a very important role in a galvanic cell: Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Then a zinc electrode is. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Web a zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge plays a very important role in a galvanic cell: Then a zinc electrode is placed. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER What Is The Function Of Zinc Electrode In A Galvanic Cell The salt bridge plays a very important role in a galvanic cell: Zinc ions in the solution are positive, and hence. Web electrons are flowing from the zinc solid metal strip to the copper strip. Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode What Is The Function Of Zinc Electrode In A Galvanic Cell Web electrons are flowing from the zinc solid metal strip to the copper strip. The salt bridge plays a very important role in a galvanic cell: Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Web a zinc sulfate solution. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From saylordotorg.github.io
Describing Electrochemical Cells What Is The Function Of Zinc Electrode In A Galvanic Cell Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From rohan-has-andersen.blogspot.com
How to Construct a Galvanic Cell RohanhasAndersen What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Then a zinc electrode is placed in the zinc sulfate solution. Web a zinc sulfate solution is floated on top of the copper sulfate solution; Web electrons are flowing from the zinc. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary What Is The Function Of Zinc Electrode In A Galvanic Cell Web because the zinc electrode in this cell dissolves spontaneously to form zn 2+ (aq) ions while h + (aq) ions are reduced to h 2 at the. Then a zinc electrode is placed in the zinc sulfate solution. Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn. What Is The Function Of Zinc Electrode In A Galvanic Cell.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell What Is The Function Of Zinc Electrode In A Galvanic Cell Web a zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge plays a very important role in a galvanic cell: Web if we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when zn 2 + ions emerge from the zinc in. Zinc ions in the solution. What Is The Function Of Zinc Electrode In A Galvanic Cell.